leak test failure autoclave|steam penetration test autoclave : chain store Chamber vacuum leak test If P2 –P1 20 mbar, probably the test has been performed while the chamber is moist If P3 – P2 13 mbar, the leaks that are evidently present and must be eliminated Today, most autoclaves have an automatic program for a routine tightness test, which is "analyzed" by the pressure measurement systems of the autoclave .
Parker Autoclave Engineers needle valves are designed for high pressure and temperature gas .For rapid system make-up, Autoclave Engineers supplies pre-cut, coned-and-threaded nipples in various sizes and lengths for Autoclave high pressure valves and fittings. In addition to the standard lengths listed in the table below, nipples are available in any custom length. Consult .
{plog:ftitle_list}
Autoclaves are widely used in biomedical laboratories at Weill Cornell Medicine (WCM but pose many hazards, including potential physical harm (e.g., heat, steam, and pressure) and .Though it is a routine lab operation, key safety measures should be taken to ensure proper autoclave operation and to avoid injuries or equipment damage: Autoclaving should be .
Some causes of Bowie-Dick test failure are poor air removal, poor steam quality, .
The Vacuum Leak Test programmed on your autoclave only measures the integrity of the sealed pressure vessel and associated piping to ensure air is not admitted to the sterilizer during the vacuum drawdowns. . Some causes of Bowie-Dick test failure are poor air removal, poor steam quality, and superheated steam conditions. Leaks in the piping .Related: Autoclave Basic Components, Leak Test used in Autoclave, Autoclave Types, biological indicator for autoclave. Important Parameters of an Autoclave. The following factors affect how an autoclave operates and needs to be maintained: Autoclave Temperature and Pressure
Leak Test Program Explained Select the required PROGRAM NUMBER that has the Leak Test program set-up via the Control Panel. . pressures and elapsed times are recorded on the autoclave printer if fitted, and logged to the autoclave log file. If a printer is not fitted, or a higher level of pressure .
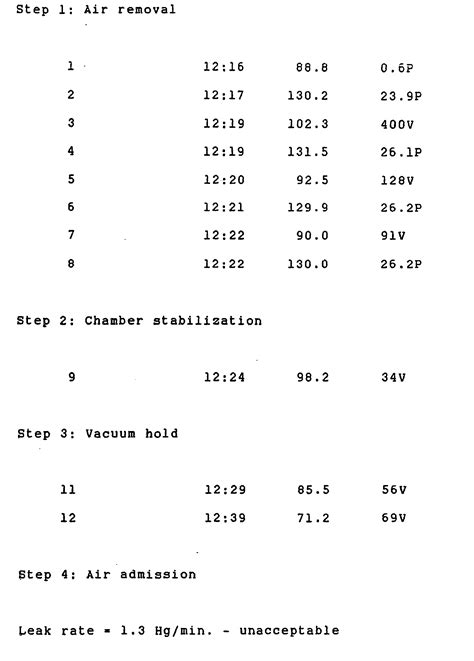
A high leak rate is only an indication of potential maintenance needs. The vacuum leak rate test is just one of the leak testing methods we use to ensure proper operation of highly specialized equipment. If you have questions surrounding how often do you run a leak test for sterilization, contact our specialists for help. Why We Need These Tests?Chamber vacuum leak test If P2 –P1 20 mbar, probably the test has been performed while the chamber is moist If P3 – P2 13 mbar, the leaks that are evidently present and must be eliminated Today, most autoclaves have an automatic program for a routine tightness test, which is "analyzed" by the pressure measurement systems of the autoclave . For tabletop sterilizers the qualification testing consists of 3 repeated cycles containing a BI in a fully loaded chamber. If a dynamic air-removal (eg, prevacuum) sterilizer has a major repair, the qualification testing must also include 3 consecutive Bowie-Dick test cycles run in an empty chamber, 1 right after the other.
The vacuum test comprises several phases which are defined in the program sequence of the autoclave:. Evacuation phase: the sterilization chamber is evacuated until the required vacuum pressure is reached. Equalization time: This is followed by an equalization time of 5 minutes to ensure that the pressure in the chamber is stable.. Measuring time: The measuring time is 10 . An autoclave is a machine that uses steam under pressure to kill harmful bacteria, viruses, fungi, and spores on items that are placed inside a pressure vessel. The items are heated to an appropriate sterilization temperature for a given amount of time.The Vacuum Leak Test is used to determine the air-tight integrity of a pre-vacuum autoclave’s chamber. Vacuum leak .Recommended Testing for Sterilizer Qualification The testing procedures are defined in the following AAMI recommended practices: • ANSI/AAMI ST79:2006/A1:2008/A2:2009, section 10, Quality control. • ANSI/AAMI ST41:2008, section 10, Quality con-trol. Susan Klacik Sterilizers: Different Repairs, Different Testing Requirements
To perform autoclave testing, autoclaves include a fully automated vacuum Leak test cycle program. This program evacuates the chamber to a set vacuum level, holds it for a specific time, and then checks the pressure again. Any increase in pressure indicates air leaking into the chamber, resulting in a failed leak test. Autoclave Vacuum Leak Testing. As the name implies, vacuum leak testing checks the air-tight integrity of the autoclave chamber. On most autoclave models, this will be a specific factory-programmed cycle that will put your autoclave through its paces and log how much “vacuum depth” is lost under specific conditions over a given time period.I had a 3000 series that would fail leak test consistently. It ended up being the surface of the door that the diaphragm gasket sits on was gashed because a previous tech had used a flathead screwdriver to remove the seal. I ended up coating a new diaphragm gasket with flange sealant and the leak never returned. This webinar will show how to identify and resolve common issues like wet loads, equilibration time failures, leak rate failures and Bowie Dick test failures for porous loads.
The Leak test will be run as an empty load. The sterilizer cycle will be set on (Leak Test.) The range of acceptable leakage is 0.0mm up to 0.9mm of pressure. The leak test will be considered a failure if the rate of leakage is 1.0mm of pressure or over. The sterilizer will be taken out of service and Biomedical Engineering will be notified.The test involves a chemical indicator test sheet inside a pack that meets the criteria specified in the ANSI/AAMI/ISO 11140 series of standards. A specified cycle is run, and the test sheet must show a uniform color change. Results that are not uniform are considered a failure.
vacuum leak test acceptance criteria
1 . dentify a steam sterilization process failure by the use of monitoring tools. 2. List 10 of the most common reasons for steam sterilization process failures. 3. Ask five questions in order to identify clues about why there was a steam sterilization failure. 4. Test the efficacy of a steam sterilizer to return into routine use. Test Questions This test exposes the autoclave’s plumbing and components to vacuum conditions and measures how much vacuum depth was lost over a given period of time. A typical Vacuum Leak Test Cycle will consist of three vacuum/pressure pulses followed by a 15-minute dwell period at deep vacuum. . or a sterilization process failure necessitating major .“The Bowie and Dick test was conceived as a test for . successful air removal for vacuum porous load sterilizers. A successful Bowie and Dick test indicates rapid and even . penetration of steam into the standard test pack or reduced test pack.” ISO 17665-1, Section 12.1.6 “If the sterilization process relies on the removal of air from the1. Leak Test – Pressing this touch pad displays OPERATING screen. Leak Test time counts down on screen. Abort touch pad is located in lower right corner. Leak Test passes through three phases: Cycle Preparation, Leak Test and Aerate. Page 56 6 — Operation Sterilization Cycle can be aborted any time during normal unit operation. If a cycle .
how to use a refractometer for aquarium
Failed Chemical Test. Possible reasons for Chemical Tests failing: Overloading Machine and improper stacking; Poorly wrapped cassettes; Autoclave malfunction; In the event of a failed chemical test run the autoclave again without any equipment in the machine. If the test passing failure was possibly due to overloading machine. If the test fails . The penetration of steam in all parts of the autoclave is essential for achieving and holding sterilization time. A Bowie dick test is done to know steam penetration within the autoclave with the help of the Bowie test pack.. While the dense bubble in the load might block steam from getting in, the bowie dick test ensures the presence of air pockets and non-condensable gases .
The leak testing equipment you use needs to have enough sensitivity and be calibrated to measure at least 100 times better than the reject rate for which you are testing. For example, if you are testing to 20 pounds per square inch (PSI) and are looking for a 2 PSI difference, but the test equipment has an analog gauge that only shows 2 PSI .
how to use a refractometer for beer brewing
sterilizer leak test parameters
Vacuum Leak Test is a test for autoclave requested by the UNI EN ISO 13060 standard. It consists in checking if a fixed increase of pressure is present between two specific periods of the process. The purpose is to check that there is no leakage in . The purpose of the present study was to establish a simple means of locating air leakage points using a gas leakage detecting aerosol in prevacuum autoclaves with Bowie-Dick test failure.The Dart Daily Air Removal Test is one of the most widely recognized pre-assembled Bowie-Dick test pack products used in monitoring healthcare steam sterilizers. It meets ANSI/AAMI/ISO 11140-4 requirements for Type 2 Air Removal Indicator Systems and operates at 270-274°F/132-134°C with an exposure time of 3.5-4.0 minutes.

Steps to take if an autoclave fails efficacy testing. If efficacy test results indicate that the autoclave failed to sterilize the indicator vial/strip, first check the autoclave record/log to make sure the correct temperature and cycle time were used. If not, adjust settings to the correct time/temperature and retest
Autoclave Testing; Cyclic Moisture Resistance; Highly Accelerated Stress Test (HAST) Moisture Sensitivity Level Testing (MSL) . which could lead to premature failure. . The Krypton-85 Leak Testing method employed by Oneida is a highly accurate technique used to measure fine and gross leak rates in high-reliability devices. It is the .
steam penetration test autoclave

Request autoclave safety training from EHS for personal operating autoclaves. Wear PPE to protect yourself from injury. Adequate PPE includes a face shield, lab coat, heat-resistant .Platelet-rich plasma (PRP) is a platelet- and growth factor-enriched solution prepared from a patient’s own blood. Injections of PRP are used to promote tissue repair and bone growth in a wide variety of medical conditions, including hair loss, osteoarthritis, . See more
leak test failure autoclave|steam penetration test autoclave